mCRPC Prior to Chemotherapy
In patients with mCRPC who had not received prior chemotherapy
ZYTIGA® + PREDNISONE ACHIEVED A MEDIAN OS OF ALMOST 3 YEARS
(34.7 MONTHS) AFTER A MEDIAN 4 YEARS (49 MONTHS) OF FOLLOW-UP*
In the final analysis of overall survival (OS) from the pivotal COU-AA-302 study, ZYTIGA® + prednisone achieved:

MEDIAN OVERALL SURVIVAL*
for ZYTIGA® + prednisone vs 30.3 months with placebo + prednisone.

IMPROVEMENT IN MEDIAN OVERALL SURVIVAL
compared with placebo + prednisone.
Co-primary endpoint — OS: hazard ratio (HR)=0.81; 95% CI: 0.70, 0.93; P=0.0033.
Co-primary endpoint — rPFS: at the prespecified radiographic progression-free survival (rPFS) analysis, median not reached for ZYTIGA® + prednisone vs a median of 8.28 months for placebo + prednisone; HR=0.425; 95% CI: 0.347, 0.522; P<0.0001.
35% of patients in the ZYTIGA® + prednisone arm were still alive vs 29% in the placebo + prednisone arm after a median follow-up of 49 months
* At a prespecified final analysis for OS, 65% (354/546) of patients treated with ZYTIGA® plus prednisone compared with 71% (387/542) of patients treated with placebo plus prednisone had died.
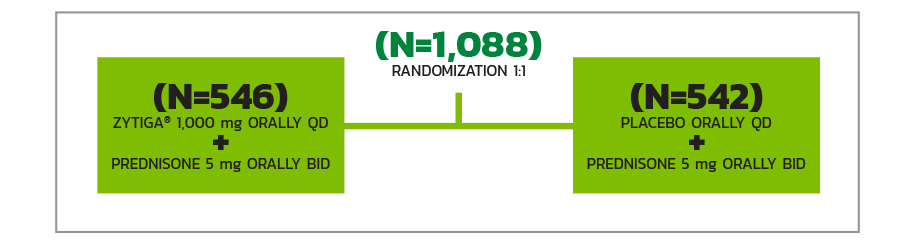
STUDY DESIGN
Randomized, double-blind, placebo-controlled, multicenter clinical trial, in patients with mCRPC who had not received prior chemotherapy (N=1,088).
All patients received a GnRH analog or had prior bilateral orchiectomy.
QD=once daily; BID=twice daily.
Patients continued treatment until:
- Protocol-Defined Radiographic Progression
OR
- Clinical Progression defined as:
- Initiation of cytotoxic chemotherapy
- Radiation or surgical treatment for cancer
- Pain requiring chronic opioids
- Eastern Cooperative Oncology Group (ECOG) performance status decline to 3 or more
OR
- Unacceptable Toxicity or Withdrawal or Death
Note:
- If the patient had radiographic progression but no unequivocal clinical progression and alternate treatment was not initiated, the patient was allowed to continue on study treatment, at the investigator’s discretion
- If the patient had unequivocal clinical progression without radiographic progression, the current standard of care was indicated. Study treatment was to be stopped and patients advised regarding available treatment options
- Study treatment was to be continued in patients who had increased prostate-specific antigen (PSA) values in the absence of radiographic or unequivocal clinical progression1
Reference: 1. Protocol for: Ryan CJ, Smith MR, de Bono JS, et al; COU-AA-302 Investigators. Abiraterone in metastatic prostate cancer without previous chemotherapy. N Engl J Med. 2013;368(2):138-148.
PATIENT POPULATION
Patients with metastatic castration-resistant prostate cancer (mCRPC) who had not received prior cytotoxic chemotherapy were included. All patients received a GnRH analog or had prior bilateral orchiectomy.
Select baseline patient demographics and disease characteristics:
- Median age=70 years1
- ECOG performance score of 0=76%; score of 1=24%
- Baseline pain assessment: 0-1 (asymptomatic)=66%; 2-3 (mildly symptomatic)=26% as defined by the Brief Pain Inventory-Short Form (worst pain over the last 24 hours)
ECOG=Eastern Cooperative Oncology Group
Select exclusion criteria included aspartate aminotransferase (AST) and/or alanine aminotransferase (ALT) >2.5X upper limit of normal (ULN), liver metastases, moderate or severe pain, opiate use for cancer pain, prior ketoconazole treatment for prostate cancer, a history of adrenal gland or pituitary disorders, and visceral organ metastases. Concurrent use of spironolactone was not allowed during the study period.1,2
References: 1. Ryan CJ, Smith MR, de Bono JS, et al; for the COU-AA-302 Investigators. Abiraterone in metastatic prostate cancer without previous chemotherapy. N Engl J Med. 2013;368(2):138-148. 2. ZYTIGA® (abiraterone acetate) Prescribing Information. Horsham, PA: Janssen Biotech, Inc.
CLINICAL STUDY RESULTS FOR MEN WITH mCRPC: COU-AA-302 Study
OVERALL SURVIVAL2
Co-primary endpoint
In the final analysis of overall survival from the pivotal COU-AA-302 study, ZYTIGA® + prednisone significantly achieved:

MEDIAN OVERALL SURVIVAL*
for ZYTIGA® + prednisone vs 30.3 months with placebo +
prednisone.

IMPROVEMENT IN MEDIAN OVERALL SURVIVAL
compared with placebo + prednisone.
Hazard ratio (HR)=0.81; 95% CI:0.70, 0.93; P=0.0033.
35% of patients in the ZYTIGA® + prednisone arm were still alive vs 29% in the placebo + prednisone arm after a median follow-up of 49 months*
* At a pre-specified final analysis for OS, 65% (354/546) of patients treated with ZYTIGA® + prednisone compared with 71% (387/542) of patients treated with placebo plus prednisone had died.
rPFS DEFINITION & ADDITIONAL RESULTS
Co-primary endpoint
At the pre-specified rPFS analysis, ZYTIGA® + prednisone achieved a 57% reduction in the risk of radiographic progression or death vs placebo + prednisone.
Median radiographic progression-free survival (rPFS) for ZYTIGA® + prednisone not reached vs 8.28 months for placebo + prednisone HR=0.425; 95% CI: 0.347, 0.522; P<0.0001.
ZYTIGA® + PREDNISONE SIGNIFICANTLY INCREASED MEDIAN rPFS VS PLACEBO + PREDNISONE*
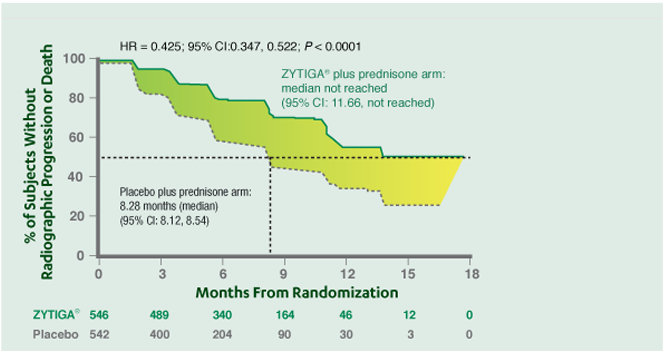
P value is derived from a log-rank test stratified by Eastern Cooperative Oncology Group (ECOG) performance status score (0 vs 1). Hazard ratio (HR) is derived from a stratified proportional hazard model. Hazard ratio <1 favors ZYTIGA®.
* Radiographically confirmed.
rPFS DEFINITION & ADDITIONAL RESULTS
Radiographic progression-free survival (rPFS) was assessed with the use of sequential imaging studies and was defined by bone scan identification of 2 or more new bone lesions with confirmation (Prostate Cancer Working Group 2 criteria) and/or modified Response Evaluation Criteria In Solid Tumors (RECIST) criteria for progression of soft tissue lesions. Analysis of rPFS utilized centrally reviewed radiographic assessment of progression.
At the prespecified rPFS analysis, 150 (28%) patients treated with ZYTIGA® + prednisone and 251 (46%) patients treated with placebo + prednisone had radiographic progression.
Adverse Reactions - The most common adverse reactions (≥10%) are fatigue, arthralgia, hypertension, nausea, edema, hypokalemia, hot flush, diarrhea, vomiting, upper respiratory tract infection, cough, and headache.
The most common laboratory abnormalities (>20%) are anemia, elevated alkaline phosphatase, hypertriglyceridemia, lymphopenia, hypercholesterolemia, hyperglycemia, and hypokalemia.
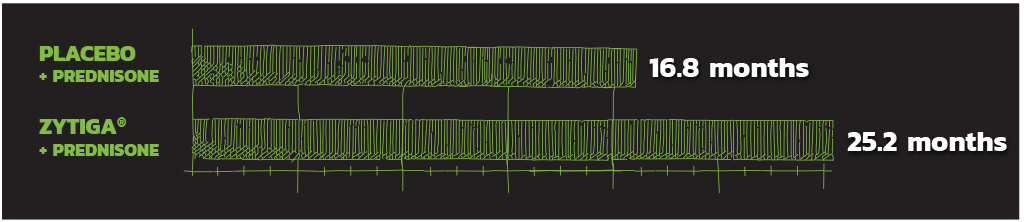
TIME TO CHEMOTHERAPY
Secondary endpoint
ZYTIGA® + PREDNISONE SIGNIFICANTLY DELAYED MEDIAN TIME TO INITIATION OF CHEMOTHERAPY.
>8 months delay in median time to initiation of chemotherapy
Median 25.2 months vs 16.8 months with placebo + prednisone. HR=0.580; 95% CI: 0.487, 0.691; P<0.0001.
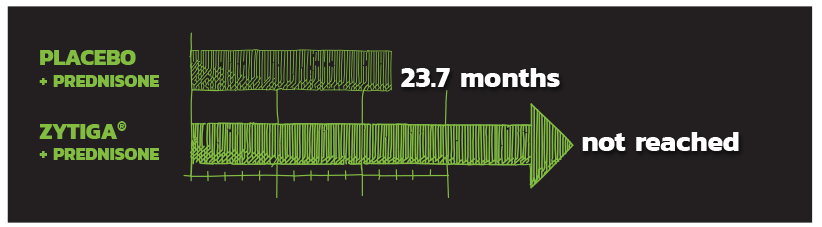
TIME TO OPIATE USE
Secondary endpoint
ZYTIGA® + PREDNISONE SIGNIFICANTLY DELAYED MEDIAN TIME TO OPIATE USE FOR PROSTATE CANCER-RELATED PAIN.
Not reached vs 23.7 months with placebo + prednisone HR=0.686; 95% CI: 0.566, 0.833; P=0.0001.
Mineralocorticoid excess: Use ZYTIGA® with caution in patients with a history of cardiovascular disease. The safety of ZYTIGA® in patients with LVEF <50% or NYHA Class III or IV heart failure in Study 1 or LVEF <50% or NYHA Class II to IV heart failure in Study 2 was not established. Closely monitor patients with cardiovascular disease. Control hypertension and correct hypokalemia before and during treatment. Monitor blood pressure, serum potassium, and symptoms of fluid retention at least monthly.
Adrenocortical insufficiency: Monitor for symptoms and signs of adrenocortical insufficiency. Increased dosage of corticosteroids may be indicated before, during, and after stressful situations.
Hepatotoxicity: Can be severe and fatal. Monitor liver function and modify, interrupt, or discontinue ZYTIGA® dosing as recommended.
Increased fractures and mortality in combination with radium Ra 223 dichloride: Use of ZYTIGA® plus prednisone/prednisolone in combination with radium Ra 223 dichloride is not recommended.
Embryo-Fetal Toxicity: ZYTIGA® can cause fetal harm. Advise males with female partners of reproductive potential to use effective contraception.
Please see full Important Safety Information.
ADVERSE REACTIONS IN >5% OF PATIENTS IN THE ZYTIGA® + PREDNISONE ARM IN COU-AA-302*
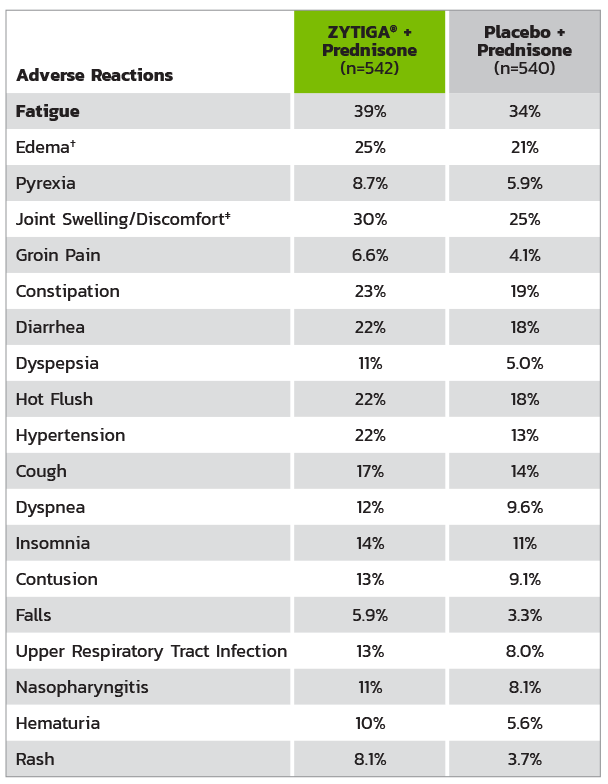
* Adverse events graded according to National Cancer Institute Common Terminology Criteria for Adverse Events (CTCAE) version 3.0.
† Includes terms: edema peripheral, pitting edema, and generalized edema.
‡ Includes terms: arthritis, arthralgia, joint swelling, and joint stiffness.
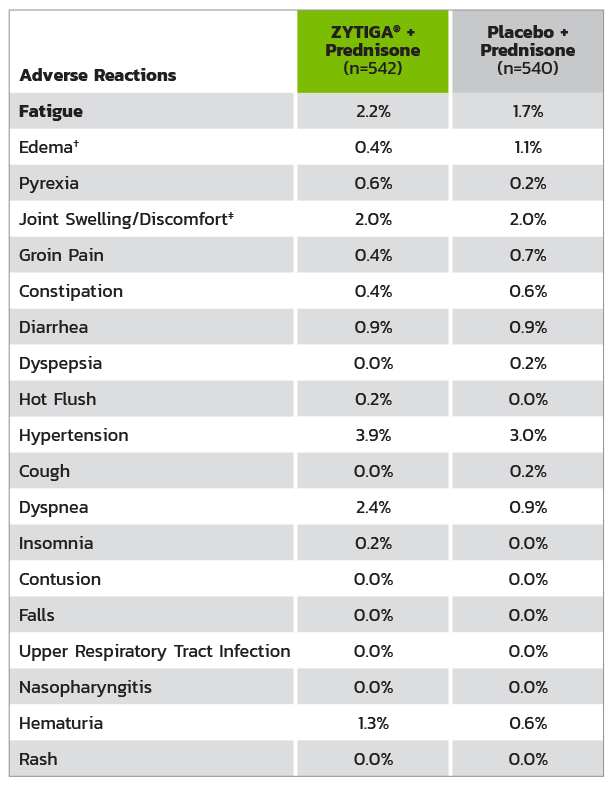
* Adverse events graded according to National Cancer Institute Common Terminology Criteria for Adverse Events
(CTCAE) version 3.0.
† Includes terms: edema peripheral, pitting edema, and generalized edema.
‡ Includes terms: arthritis, arthralgia, joint swelling, and joint stiffness.
References: 1. Ryan CJ, Smith MR, de Bono JS, et al; for the COU-AA-302 Investigators. Abiraterone in metastatic prostate cancer without previous chemotherapy. N Engl J Med. 2013;368(2):138-148. 2. Ryan CJ, Smith MR, Fizazi K, et al; for the COU-AA-302 Investigators. Abiraterone acetate plus prednisone versus placebo plus prednisone in chemotherapy-naive men with metastatic castration-resistant prostate cancer (COU-AA-302): final overall survival analysis of a randomized, double-blind, placebo-controlled Phase 3 study. Lancet Oncol. 2015;16(2):152-160.
Find out how to dose and monitor the use of ZYTIGA® (abiraterone acetate) for your patients.


