mCRPC Following Chemotherapy Study
STUDY DESIGN
Randomized, double-blind, placebo-controlled, multicenter clinical trial in patients with mCRPC who received prior docetaxel therapy (N=1,195).
All patients received a GnRH analog or had prior bilateral orchiectomy. Selected exclusion criteria included prior ketoconazole treatment for prostate cancer and a history of cardiovascular disease: LVEF <50% or New York Heart Association (NYHA) Class III or IV heart failure. Concurrent use of spironolactone was not allowed during the study period.b
QD=once daily; BID=twice daily.

In this clinical study, patients were to continue until disease progression, initiation of new treatment, unacceptable toxicity, or withdrawal.
All of the following criteria were to be met prior to discontinuation due to progression1:
- Increased PSA* as defined by the PSA Working Group (PSAWG) (25% increase over baseline, with a minimum PSA increase of 5 ng/mL)
- Radiographic progression (progression on bone scans or soft-tissue disease progression)
- Symptomatic or clinical progression defined by pain progression, development of skeletal-related events, increase in prednisone or prednisolone dose, or change in corticosteroid to treat prostate cancer–related signs and symptoms
* PSA=prostate-specific antigen.
Reference: 1. Protocol for: de Bono JS, Logothetis CJ, Molina A, et al; for the COU-AA-301 Investigators. Abiraterone and increased survival in metastatic prostate cancer. N Engl J Med. 2011; 364(21): 1995-2005.
DURATION OF TREATMENT
At the updated analysis for overall survival, the median duration of treatment in patients with mCRPC who received prior docetaxel therapy was1:
ZYTIGA® + Prednisone
7.4 MONTHS
Placebo + Prednisone
3.6 MONTHS
PATIENT POPULATION
All patients received a GnRH analog or had prior bilateral orchiectomy.
- Median age=69 years2
- ECOG performance score of 0 to 1=89%
- Brief Pain Inventory-Short Form score of ≥4 (patients reported worst pain over the previous 24 hours)=45%
- Bone metastases=90%; visceral involvement=30%
- The most common sites of visceral disease were the lungs (42%) and liver (33%). Other sites included prostate mass, bladder, and adrenal glands3
- Prior cytotoxic chemotherapy: 1 regimen=70%; 2 regimens=30%
- Radiographic evidence of disease progression=70%; PSA-only progression=30%
ECOG=Eastern Cooperative Oncology Group;
PSA=prostate-specific antigen.
All patients received a GnRH analog or had prior bilateral orchiectomy. Selected exclusion criteria included prior ketoconazole treatment for prostate cancer and a history of cardiovascular disease: LVEF <50% or New York Heart Association (NYHA) Class III or IV heart failure. Patients were not eligible if AST and/or ALT ≥2.5X ULN in the absence of liver metastases. Patients with liver metastases were excluded if AST and/or ALT >5X ULN. Concurrent use of spironolactone was not allowed during the study period.1
Reference: 1. de Bono JS, Logothetis CJ, Molina A, et al; for the COU-AA-301 Investigators. Abiraterone and increased survival in metastatic prostate cancer. N Engl J Med. 2011;364(21):1995-2005.
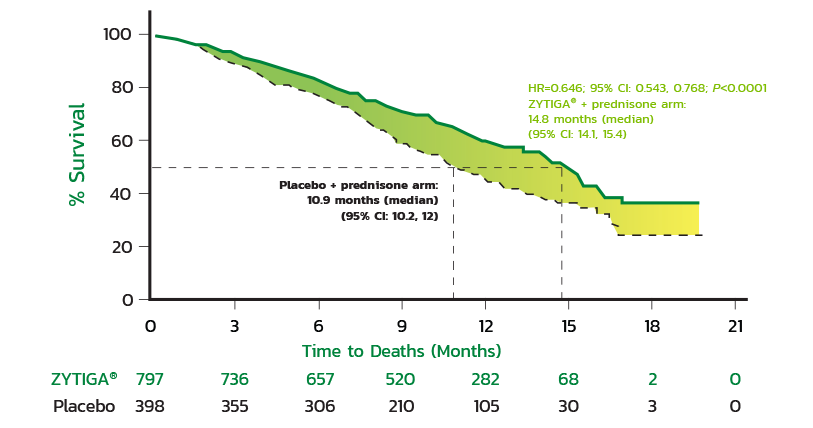
CLINICAL STUDY RESULTS
Median Overall Survival
The primary survival analysis showed a 3.9-month difference in median OS for ZYTIGA® + prednisone compared with placebo + prednisone (14.8 months vs 10.9 months, respectively) (HR=0.646; 95% CI: 0.543, 0.768; P<0.0001).
4.6 MONTHS IMPROVEMENT IN MEDIAN OVERALL SURVIVAL (OS) AT THE UPDATED ANALYSIS
Median OS: 15.8 months with ZYTIGA® plus prednisone vs 11.2 months with placebo plus prednisone HR=0.740; 95% CI: 0.638, 0.859; P<0.0001.1
Kaplan-Meier Survival Curve
Kaplan-Meier curves of patients treated with either ZYTIGA® + prednisone or placebo + prednisone (primary survival, intent-to-treat-analysis).
HR=0.646; 95% CI: 0.543, 0.768; P<0.0001.
Mineralocorticoid excess: Closely monitor patients with cardiovascular disease. Control hypertension and correct hypokalemia before treatment. Monitor blood pressure, serum potassium, and symptoms of fluid retention at least monthly.
Adrenocortical insufficiency: Monitor for symptoms and signs of adrenocortical insufficiency. Increased dosage of corticosteroids may be indicated before, during, and after stressful situations.
Hepatotoxicity: Can be severe and fatal. Monitor liver function and modify, interrupt, or discontinue ZYTIGA® dosing as recommended.
Please see full Important Safety Information.
ADVERSE REACTIONS IN PATIENTS IN THE ZYTIGA® + PREDNISONE ARM IN COU-AA-301*
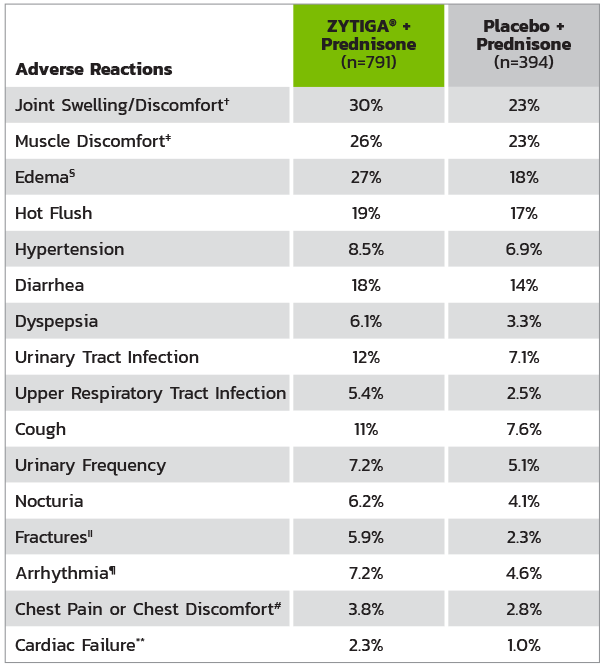
* Adverse events graded according to National Cancer Institute Common Terminology Criteria for Adverse Events (CTCAE) version 3.0.
† Includes terms arthritis, arthralgia, joint swelling, and joint stiffness.
‡ Includes terms muscle spasms, musculoskeletal pain, myalgia, musculoskeletal discomfort, and musculoskeletal stiffness.
§ Includes terms edema, edema peripheral, pitting edema, and generalized edema.
II Includes all fractures with the exception of pathological fracture.
¶ Includes terms arrhythmia, tachycardia, atrial fibrillation, supraventricular tachycardia, atrial tachycardia, ventricular tachycardia, atrial flutter, bradycardia, atrioventricular block complete, conduction disorder, and bradyarrhythmia.
# Includes terms angina pectoris, chest pain, and angina unstable. Myocardial infarction or ischemia occurred more commonly in the placebo arm than in the ZYTIGA® arm (1.3% vs 1.1%, respectively).
** Includes terms cardiac failure, cardiac failure congestive, left ventricular dysfunction, cardiogenic shock, cardiomegaly, cardiomyopathy, and ejection fraction decreased.
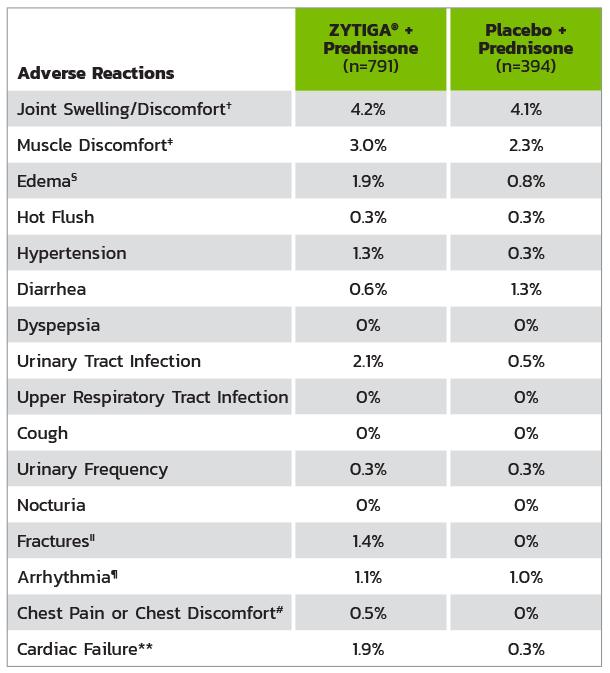
* Adverse events graded according to National Cancer Institute Common Terminology Criteria for Adverse Events (CTCAE) version 3.0.
† Includes terms arthritis, arthralgia, joint swelling, and joint stiffness.
‡ Includes terms muscle spasms, musculoskeletal pain, myalgia, musculoskeletal discomfort, and musculoskeletal stiffness.
§ Includes terms edema, edema peripheral, pitting edema, and generalized edema.
II Includes all fractures with the exception of pathological fracture.
¶ Includes terms arrhythmia, tachycardia, atrial fibrillation, supraventricular tachycardia, atrial tachycardia, ventricular tachycardia, atrial flutter, bradycardia, atrioventricular block complete, conduction disorder, and bradyarrhythmia.
# Includes terms angina pectoris, chest pain, and angina unstable. Myocardial infarction or ischemia occurred more commonly in the placebo arm than in the ZYTIGA® arm (1.3% vs 1.1%, respectively).
** Includes terms cardiac failure, cardiac failure congestive, left ventricular dysfunction, cardiogenic shock, cardiomegaly, cardiomyopathy, and ejection fraction decreased.
References: 1. Fizazi K, Scher HI, Molina A, et al. Abiraterone acetate for treatment of metastatic castration-resistant prostate cancer: final overall survival analysis of the COU-AA-301 randomized, double-blind, placebo-controlled Phase 3 study. Lancet Oncol. 2012;13(10):983-992. 2. de Bono JS, Logothetis CJ, Molina A, et al; for the COU-AA-301 Investigators. Abiraterone and increased survival in metastatic prostate cancer. N Engl J Med. 2011;364(21):1995-2005. 3. Goodman OB, Flaig TW, Mulders PFA, et al. Exploratory analysis of the visceral disease subgroup in a Phase 3 study of abiraterone acetate in metastatic castration-resistant prostate cancer. Prostate Cancer Prostatic Dis. 2014;17:34-39.
Find out how to dose and monitor the use of ZYTIGA® (abiraterone acetate) for your patients.
